| ACE-inhibitory activity |
The peptide PRY exhibited potent Angiotensin-Converting Enzyme (ACE) (EC 3.4.15.1) inhibitory activity with an IC50 value of 97.59 μM, and its ACE-inhibitory pattern was shown by Lineweaver–Burk plots to be a competitive inhibition pattern.
Molecular docking between patatin peptides (WG and PRY) and ACE were performed. The best docking position is presented in Fig. 1A & B with Eele values of −60.76 and −40.61 kJ/mol, Evdw values of −4.51 and −2.93 kJ/mol and Epot values of −58.93 and −47.70 kJ/mol, respectively. Four hydrogen bonds were observed with ACE- WG interaction (Arg124, Glu123, Glu411 and Tyr523), and six (Arg124, Asp358, Arg522, Glu123 and Tyr523) for ACE-PRY. The results suggest that PRY had greater interactions within the ACE, which may have contributed to greater inhibitory potency when compared to WG. The results are in agreement with the lower ACE IC50 values of PRY when compared to WG. However, there was no direct interaction observed between PRY and the Zn(II) with the active site of ACE. In contrast, WG formed H-bonds with Zn(II) (Fig. 1A), which could have provoked distortion of Zn(II) structure and produced impaired ACE activity.
In terms of ACE docking, the crystal structure of human ACE was obtained from Protein Data Bank (1O8A.pdb).
 | 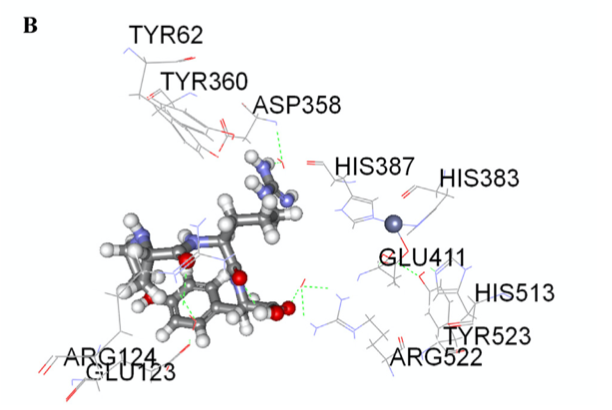 | Fig.1. Molecular docking of WG (A) and PRY (B) at ACE activesites. Hydrogen bonds are represented by green dotted lines and other amino residues are automatically generated from Accelrys DS 2.5 Visualizer software.
|
|
| Specific target protein(s) |
Specific Target Protein(s):
Angiotensin-converting enzyme |