| Renin-inhibitory activity |
The tripeptide PRY exhibited potent Renin (EC:3.4.23.15) inhibitory activity with an IC50
value of 95.33 uM, and its renin inhibitory pattern was shown by Lineweaver–Burk plots to be a mixed-type inhibition pattern.
The docking simulations for peptide interactions with renin are displayed in Fig. 1A & B with Eele values of −44.86 and −82.65 kJ/mol, Evdw values of −1.53 and −7.04 kJ/mol and Epot values of −40.32 and −61.56 kJ/mol, respectively. WG interacted with three of renin residues (Arg157, Thr7 and Glu17), while PRY displayed interactions with six residues (Lys308, Ala325, Ser3, Glu176, Glu17 and Ile5). The higher number of H-bonds found in PRY with renin residues might account for the higher inhibitory potency when compared to WG. The molecular docking data are consistent with the higher renin-inhibitory activity of PRY when compared to WG. For renin docking, a crystal structure of human renin was obtained from Protein Data Bank (2V0Z.pdb).
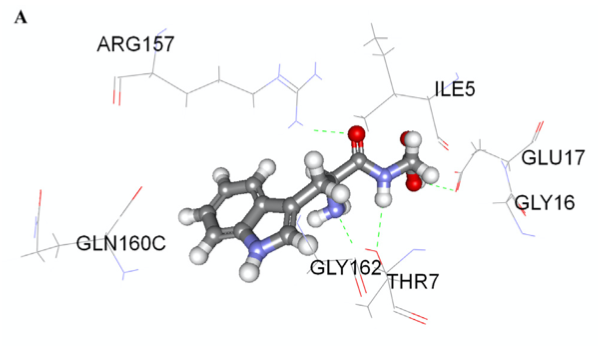 | 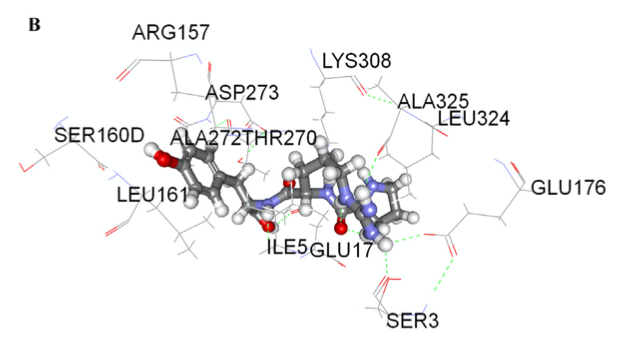 | Fig. 1. Molecular docking of WG (A) and PRY (B) at renin active sites.
Hydrogen bonds are represented by green dotted lines and other amino
residues are automatically generated from Accelrys DS 2.5 Visualizer
software.
|
|
| Specific target protein(s) |
Specific Target Protein(s):
Renin |